All about batteries
Essential to our daily lives, batteries power a multitude of devices, from telephones to electric cars. But how do they work? What different types exist? And above all, how can they be properly recycled? Find out everything you need to know about batteries, from their composition to their second life, including the most suitable recycling solutions.
What is a battery?
A battery is a device that stores electrical energy and then releases it. It does this by converting the energy of a chemical reaction into electricity. This process causes the battery to discharge, but unlike batteries, this discharge is reversible. Batteries are therefore rechargeable.
How do batteries work?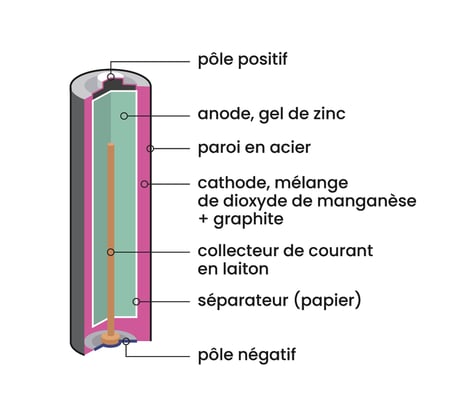
Like a battery, a battery is made up of three components:
- Two electrodes: cathode (positive pole) and anode (negative pole).
- An electrolyte: a conductive solution in which the electrodes are immersed.
Batteries generally consist of :
- Plastic.
- Paper (case).
- Various types of metal: lithium, nickel, cadmium, manganese, lead...
These materials differ according to battery type.
When the circuit is closed, electrons flow from the anode to the cathode, generating an electric current that powers the connected device. A battery operates on the same principle as a cell: a chemical reaction between the positive and negative poles.
In the case of a battery, this chemical reaction is reversible: by recharging it, the process is reversed and the battery can be reused.
How batteries work: understand it all in 2 minutes →
What are the different types of battery?
There are many different types of battery, each designed to meet specific needs. Capacity, lifespan, cost... their performance varies according to the technology used.

1. Lithium batteries: Lithium-ion (Li-ion) or Lithium polymer
Introduced in the 1980s, lithium batteries have become a benchmark in energy storage. Based on an electrochemical reaction, they are gradually replacing nickel-cadmium batteries.
- Composition: lithium, cobalt, nickel, manganese, aluminum/copper (electrodes)
- Multiple advantages: High capacity, long life, lighter, less bulky, low self-discharge.
- Limitations: Risk of ignition, use of rare metals, higher production costs.
- Applications: Power tools (drills, screwdrivers and other professional equipment), electronic devices (smartphones, computers, tablets, cameras, etc.), energy storage solutions (stationary batteries, domestic solar panels).
2. Nickel Cadmium (NiCd) batteries
Nickel Cadmium (NiCd) batteries are based on proven technology that has been in use for several decades. Less common today, they are still preferred for certain industrial and safety applications.
- Composition: steel, nickel, cadmium, potash (electrolyte).
- Advantages: Reliability, robustness, excellent resistance to extreme temperatures, high and stable current, long life, tolerance to deep discharges, fast and efficient recharging.
- Limitations: Memory effect, presence of cadmium (toxic substance), environmental impact.
- Applications: Hand-held power tools, industrial and safety applications (emergency systems, emergency lighting, professional equipment).
Nickel Metal Hydride (NiMH) batteries are an alternative to Nickel Cadmium batteries. They replace cadmium with a hydride alloy based on nickel, cobalt and rare earths. Less polluting, they are now the preferred choice for many everyday wireless devices.
Find out more about recycling Lithium-ion batteries →
3. Lead-acid batteries
Lead-acid batteries were the first battery technology to be developed. Despite the emergence of new technologies, they remain indispensable in certain sectors.
- Composition: Lead (electrodes), sulfuric acid (electrolyte), plastics (case).
- Advantages: High current, proven and robust technology, affordable cost, decent endurance.
- Limitations: Weight and bulk, sensitivity to deep discharge, environmental impact.
- Applications: Automotive (starter batteries for internal combustion engines), inverters and alarms, mobile medical equipment, automated gates and security systems.
Energy storage is constantly evolving, and lithium-ion batteries are no longer the only solution for the future. All over the world, research is exploring innovative, greener and more efficient alternatives, shaping the future of energy storage.
Innovation and batteries: the latest advances →
What batteries are used in electric cars?
Lithium ion batteries play a key role in the development of electric vehicles. Three main variants are used in Europe:
- Lithium-iron-phosphate (LFP): robust, safe and less expensive.
- Nickel-Manganese-Cobalt (NMC ): balance between performance, autonomy and longevity.
- Nickel-Cobalt-Aluminium (NCA): high energy density, ideal for long distances.
The growth of the automotive market is accelerating research into the optimization of these batteries, with innovations such as lithium-iron phosphate batteries, which offer greater thermal stability and longer life.
Where to dispose of used batteries?
Batribox collection points are the solution for recycling your small batteries and used portable accumulators:
- Nickel-Cadmium (NiCd)
- Nickel-Metal Hydride (NiMh)
- Lithium (Lithium-ion, Lithium-polymer)
- Lead (except automotive starter batteries and batteries over 5 kilos)
- Button cell batteries
Industrial batteries (over 5 kg) and batteries for small-scale electric mobility (bicycles, scooters, etc.) are also collected by Batribox via specific channels.
More information on the e-mobility voluntary channel →
More information on the collection of industrial batteries weighing over 5 kg →
Batribox collection points are located throughout France:
- In stores selling batteries (mandatory free collection).
- In supermarkets and DIY stores, equipped with suitable containers.
- At waste collection centers, for secure collection and optimized recycling.
🔍 F ind a collection point near you and give your batteries a second life!
Once collected, batteries are sorted into 4 main categories before being recycled: nickel, metal hydride, rechargeable lithium and lead. The metals they contain (aluminum, copper, cobalt, lithium...) are then recovered to limit the extraction of new resources.
This practice is part of a circular economy approach. Rather than recycling them immediately, reusing used batteries :
- Extends their useful life.
- Limits waste.
- Preserves natural resources.
- Reduces dependence on strategic materials.
It's also an economic lever: by maximizing their lifespan, we optimize costs and encourage more sustainable energy management.
After an initial cycle of use, batteries can be given a second life thanks to two innovative solutions: remanufacturing and reuse.
- Remanufacturing optimizes performance by rebalancing cells, replacing defective ones or even repairing batteries.
- Reuse involves giving a new purpose to cells that are still functional, notably for energy storage, industry or certain electric vehicles.
These alternatives prolong the use of batteries, reduce waste and contribute to more sustainable resource management.
All about second life →
All about recycling →
Find your nearest collection point!
Research scope